Extra virgin olive oil: What are we talking about?…an advanced Chemistry lesson by the MONOGRAM Team
It’s hard to talk about the benefits of olive oil without referencing its important role in the Mediterranean diet. This way of eating consistently ranks as the #1 diet on the planet for your health according to nutritionists, researchers, and doctors.
Olive oil is the staple ingredient running through all Mediterranean cuisine. Not the cheap, blend refined oil, but the beautiful Extra virgin olive oil in its least processed form; first cold-pressed. What makes it so nutritious to consume? The answer is hidden in Extra Virgin Olive Oil “Chemistry”…..
Let’s have an advanced chemistry lesson …..
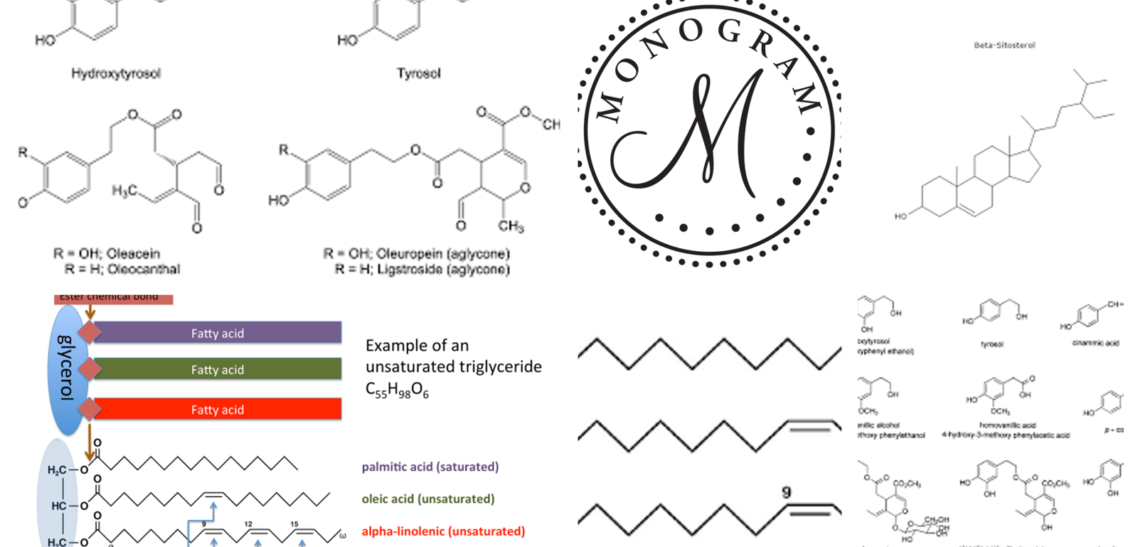
Olive oil mostly consists of triglycerides (98–99%) and contains primarily monounsaturated fatty acids (MUFAs) in the form of omega-9 oleic acid (C18:1); according to the International Olive Oil Council, its concentration must range from 55 to 83% of total fatty acids.
Olive oil also contains other MUFAs, such as omega-7 palmitoleic acid (C16:1), ranging from 0.3 to 3.5%, and traces of gadoleic/9-eicosenoic (C20:1 ω-11, 0.4%) and heptadecenoic acid (C17:1, 0.3%).
Extra virgin olive oil also contains polyunsaturated fatty acids (PUFAs) including linoleic acid (C18:2, ω-6) and α-linolenic acid (C18:3, ω-3), between 3 and 19% and 0.11 and 1.0%, respectively. EVOO’s lipid profile and high ω6/ω3 ratio have been linked to its protective effects on cardiovascular (CV), autoimmune and inflammatory disorders, but also its anti-thrombotic and blood pressure regulatory qualities, and ensuring oxidative stability for long shelf life (1–3).
Saturated fatty acids participate in the EVOO fatty acid profile: palmitic acid (C16:0, 7.8–17.3%), stearic acid (C18:0, 0.2–3.2%), arachidic acid (C20:0, 0.7%), margaric acid (C17:0, 0.3%), behenic acid (C22:0, 0.2%), lignoceric acid (C24:0, 0.2%), and myristic acid (C14:0, 0.03%).
The minor fraction of EVOO comprises substances responsible for its biological properties and sensory attributes (color, odor, flavor, taste, and aftertaste), primarily present in the mature drupe pulp and pits which are dissolved in the oil via natural or technological processes. Lower quantities of squalene (3–6 g/kg) and phytosterols (β-sitosterol, campesterol, and stigmasterol, in free and esterified forms) (0.8–2.6 g/kg) are present in EVOO. Finally, soluble vitamins (β-carotene and tocopherols), pigments (carotenes and chlorophyll), alcohol triterpene, and especially polyphenols are present in minor quantities.
Phenolic compounds include about 30 molecules from different chemical classes: phenolic alcohols, such as hydroxytyrosol (HT) and tyrosol (Tyr), phenolic acids, flavones, lignans, and secoiridoids. The latter group represents the largest fraction. The principal ones are the aglycone forms of oleuropein and ligstroside, the dialdehydic forms of their decarboxymethylated derivatives, known as oleacein and oleocanthal (Supplementary Table 1). In OO, the content of phenolic compounds ranges from 50 to 1000 mg/kg. The secoiridoids act as natural antioxidants protecting EVOO against autoxidation during storage and are responsible for its bitter and pungent qualities. Much evidence indicates that EVOO’s phenolic compounds can exert biological activities due to their antioxidant, anti-inflammatory, and chemo-preventive properties (4, 5).
In humans, Tyr and HT intestinal absorption occurs in a dose-dependent way with a percentage ranging from 40 to 95% and is strictly dependent on the polarity of their chemical structure (6, 7). Part of these polyphenols, in particular aglycone secoiridoids, can be hydrolyzed at the gastric level, with a time-dependent process, transforming into free Tyr and HT (8); the glycosylated forms do not suffer hydrolysis processes and, together with other polyphenols, pass through the small intestine where they are absorbed by enterocytes via a bidirectional passive diffusion mechanism. Once absorbed, polyphenols undergo phase II transformation metabolism, which substantially reduces their bioavailability. The most represented metabolites in plasma are the O-glucuronidated forms of Tyr and HT (9) and, to a lesser extent, homovanillic acid, homovanillic acid sulfate, and HT acetate sulfate (10). Both the unmodified forms and the metabolites of the polyphenol subclasses are ubiquitously distributed in the organism, depositing, in a concentration-dependent way, in certain organs, such as the brain, liver, and kidneys (11).
The clearance of polyphenols and their metabolites essentially occurs via kidney excretion (12).
Based on health studies, in 2011 the European Food Safety Authority (EFSA) authorized a functional health claim on EVOO polyphenols that they “contribute to the protection of blood lipids from oxidative stress.” This benefit emerges with a minimum concentration of 5 mg of HT and its derivatives in 20 g of EVOO (13). Nevertheless, this is a contested point. Originally, Regulation (EC) No 1924/2006 included several health claims for OO polyphenols [for details see European Community, (14)]. In 2011, EFSA was asked about these claims and concluded: “that a cause and effect relationship has been established between the consumption of OO polyphenols and protection of low-density lipoproteins (LDL) particles from oxidative damage” (13). In 2012, the European Council updated the regulation to implement this opinion (15). Since then, Extra Virgin Olive Oil has been elevated to the heavens…
Proudly created by MONOGRAM Team for you
References
1. Mariotti M, Peri C. The composition and nutritional properties of extra-virgin olive oil. In: C Peri editor. The Extra-Virgin Olive Oil Handbook. Hoboken, NJ: John Wiley & Sons Ltd (2014). p. 21–34.
2. Sánchez-Villegas A, Sánchez-Tainta A. The Prevention of Cardiovascular Disease through the Mediterranean Diet. Cambridge, MA: Academic Press (2018). p. 59–87.
3. Lombardo L, Grasso F, Lanciano F, Loria S, Monetti E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds. 1st ed. (Vol. 57). Amsterdam: Elsevier (2018).
4. Foscolou A, Critselis E, Panagiotakos D. Olive oil consumption and human health: a narrative review. Maturitas. (2018) 118:60–6. doi: 10.1016/j.maturitas.2018.10.013,
5. Emma MR, Augello G, Di Stefano V, Azzolina A, Giannitrapani L, Montalto G, et al. Potential uses of olive oil secoiridoids for the prevention and treatment of cancer: a narrative review of preclinical studies. Int J Mol Sci. (2021) 22:1234. doi: 10.3390/ijms22031234
6. Weinbrenner T, Fitó M, Farré Albaladejo M, Saez GT, Rijken P, Tormos C, et al. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp Clin Res. (2004) 30:207–12.
7. Manna C, Galletti P, Maisto G, Cucciolla V, D’Angelo S, Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. (2000) 470:341–4. doi: 10.1016/s0014-5793(00)01350-8
8. Corona G, Tzounis X, Assunta Dessì M, Deiana M, Debnam ES, Visioli F, et al. The fate of olive oil polyphenols in the gastrointestinal tract: implications of gastric and colonic microflora-dependent biotransformation. Free Radic Res. (2006) 40:647–58. doi:10.1080/10715760500373000
9. Caruso D, Visioli F, Patelli R, Galli C, Galli G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism. (2001) 50:1426–8. doi: 10.1053/meta.2001.28073
10. Rubió L, Valls RM, Macià A, Pedret A, Giralt M, Romero MP, et al. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. (2012) 135:2922–9. doi: 10.1016/j.foodchem.2012.07.085
11. López de las Hazas MC, Rubió L, Kotronoulas A, de la Torre R, Solà R, Motilva MJ. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol Nutr Food Res. (2015) 59:1395–9. doi: 10.1002/mnfr.201500048
12. Lozano-Castellón J, López-Yerena A, Rinaldi de Alvarenga JF, Romero Del Castillo-Alba J, Vallverdú-Queralt A, Escribano-Ferrer E, et al. Health-promoting properties of oleocanthal and oleacein: two secoiridoids from extra-virgin olive oil. Crit Rev Food Sci Nutr. (2020) 60:2532–48. doi: 10.1080/10408398.2019.1650715
13. EFSA. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/200. EFSA J. (2011) 9:2033.
14. European Community.Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Germany: European Union (2006).
15. 28. European Community.Council Regulation No. 432/2012 of 16 May 2012 Establishing a list of Permitted Health Claims Made on Foods, other than those Referring to the Reduction of Disease Risk, to Children’s development, Health. Germany: European Union (2012).
